
OpenWater is a global award winning manufacturing consultancy with its expertise in medical devices design, compliance and manufacture, from conception to market and, for the device lifecycle to disposal.
We get actively involved in plant design, validation, manufacturing processes, Quality Management Systems, compliance, regulatory strategies, marketing applications and, we work hands on with our clients to ensure the shortest lead times to market.
We are very aware of regulatory requirements and price pressures and, we understand the need to combine innovation with responsible manufacturing by adding value to your business
OpenWater Hydrocolloid Formulas
In today's competitive marketplace, buying in can be expensive. It often comes with concerns about distribution and supply continuity, offering little flexibility and control. This can ultimately lead to additional costs, including lost sales, customers, and revenue.
When there are delays in delivery, the impact on patients is immeasurable.
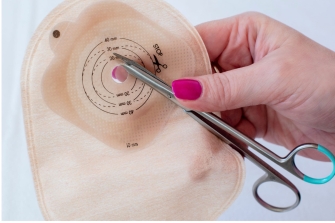
We provide manufacturers with a practical solution that allows for vertical integration that increases control over the process. With over forty year’s experience, we supply hydrocolloid formulas, along with the knowledge and processing expertise, so you can take complete ownership and eliminate the aforementioned risks.
OpenWater's ostomy adhesive hydrocolloid formulas, offer fluid absorption and gelation properties with an adhesive and cohesive structure. This elastomeric adhesive is pressure-sensitive, developing immediate adhesion upon light pressure application.

OpenWater's hydrocolloids play a crucial role in device performance by supporting the ostomy bag and protecting the peristomal skin from exposure to stomal output. The adhesive's composition is vital for the device's overall effectiveness, enabling it to adhere safely to the skin. The gelling agents within the hydrocolloid absorb moisture from the user's skin during the baseplate's wear time.
Overall, OpenWater's hydrocolloid formulas provide patients with the essential confidence and security required from every ostomy device.

OpenWater International Ltd
OpenWater boasts a unique combination of skillsets that complement each other to provide clients with the complete ability to take a new product concept through to full realisation and market placement.
Our experience has been mainly focused on the devices industry, mostly in high margin, single use devices such as wound care, general surgical devices, ophthalmics and ostomy manufacture, including the formulation and manufacture of hydrocolloid.
We are expert in assisting Start Ups and we provide specialist help for small businesses, where we have strength and experience in bringing a small company's products from the design stage into full production. We install full quality assurance systems, advise on applicable regulatory requirements and, provide machinery design.
If you are a medium or large manufacturer with a particular project to be completed, or need to call in some specialists for a while, we are willing to help.
We are happy to roll up our sleeves and join you on the shop floor to make product, design equipment and train staff. We will give you no nonsense, sound knowledge to save you time and costs.
We can advise on all aspects of medical devices design, materials, machinery, tooling, processes, factory layout, work flow and value engineering.
We supply services in Regulatory Affairs and advise on all classes of medical devices design, management systems, global marketing compliance, marketing applications, tenders, legalistion of documents, due diligence and lean regulatory strategies.
Nick Shelley is the Manufacturing Consultant who advises on all aspects of medical devices design, materials, machinery, tooling, processes, factory layout, work flow and value engineering.
Get in touch today to find out how Nick can advise your company through every stage of the manufacturing process, to give you the freedom and confidence to expand without limits.
Email NickShelley@OpenWater.uk.com
Joanne Davies is the Lead Consultant in Regulatory Affairs and advises on all classes of medical devices design, compliance, Quality management systems, global marketing applications, tenders, legalistion of documents, due diligence and lean regulatory strategies.
Email JoanneDavies@OpenWater.uk com to ensure your route to market is efficient, compliant and the most cost effective. But above all, your devices are safe and effective for the patient and/or user and the environment.
At OpenWater we have more than sixty years of knowledge and diverse experience in the manufacturing world. We have built up access to a wide distribution network by supplying consultancy services to global markets.
... from concept to market ...
... plain sailing with OpenWater!
Partners
Services
|
|
||
| Factory | Manufacturing facility design layout | |
| Materials and manufacturing process selection | ||
| Tooling design | ||
| Process introduction | ||
| Planning and installation | ||
| Business continuity planning | ||
| Manufacturing data restructure | ||
| Production and process validation | ||
| Cleanroom | Design, build and validation | |
| Machinery | Small machinery design, build and validation |
|
| Hydrocolloid | Formulation and manufacture | |
| Product Design and Development | Design for manufacture and assembly |
|
| Product development process | ||
| Project management | ||
| Engineering drawings | ||
| Devices risk management | ||
| Technical documentation | ||
| CE marking | ||
| Raw Materials | Materials and manufacturing process selection | |
| Specifications | ||
| Biocompatibility studies | ||
| Supplier partnership and development | ||
| Processes | Quality Management System design | |
| Quality Management System implementation | ||
| Workflow and automation | ||
| Build or restructure | ||
| Quality Assurance | Quality Management Systems design and implementation | |
|
Quality Management Systems
Dependent on the client’s regulatory pathway we can design and supply all the required Quality Management System documentation. We can create this around any industry preferred standard. The most common standard globally for medical devices is 13485, but 9001 is also acceptable as is any other Quality Management System of the client’s choice
We design the Quality Manual which illustrates the structure and function of all personnel and processes within the business that are associated with the Quality of the product. This can be supplied to anyone in the supply chain as a marketing tool and for conformity assessment activities including audits, certification and global marketing applications
We include all record templates, procedures and processes required to satisfy compliance requirements in more than 85 countries |
||
| Quality Management Systems audits | ||
| Proccedures and forms | ||
| Test report writing | ||
| QMS Representative | ||
| Notified body | ||
| Accelerated shelf life studies | ||
| Systems and software validation | ||
| Calibration systems and services | ||
| Regulatory Affairs | Regulatory strategy | |
| Interim management | ||
| Design dossier compliance review | ||
| Technical file compliance review | ||
| Global marketing applications and reviews | ||
| Notified body | ||
| Global marketing submissions | ||
| Tenders | ||
| Person responsible for regulatory affairs | ||
| ISO Certification | ||
| 13485, 9001, 14971, 60601, 10993, (EU) 2017/745, 3071/746, CFR 820 | ||
| Global Competent Authority Representative | ||
| Training |
Devices risk management |
|
|
Risk management of medical devices is critical to determine all compliance requirements are implemented, during research and development, during the production stage and post market. It therefore includes all design and production process changes as regulatory pathways are determined, mapped and implemented
The Risk Management Process from OpenWater is based on ISO 14971 - Application of Risk Management to Medical Devices or any other relevant technical industry standard of your choice
OpenWater’s Risk Management Process includes the following elements with regard to Class I medical devices
Risk analysis, risk evaluation, risk control, risk verification, risk evaluation, overall residual risk evaluation and production and post production information
Risk Identification OpenWater’s Risk Identification includes the identification of hazards for Safety, Product Realisation, General Safety, Biological Hazards and Post Market Surveillance
What We Supply We supply the Risk Management Procedure and all documentation including a final Risk Management Report that is compliant with the regulatory requirements of more than 86 countries
Risk Management Training OpenWater can supply training to satisfy Quality Management System training requirements that includes video call support to complete the paperwork and all risk associated tasks |
||
| Legal | Apostille | |
| Legalisation of documents | ||
| Global Embassy Representative | ||
| Patent advice, patents and Intellectual Property services | ||

Contact
Head Office
OpenWater International Ltd
Unit D,
Otehall Farm Busines Park,
Janes Lane,
Burgess Hill,
West Sussex,
England
RH15 0SR
Tel +44 (0)7704 426 371
Email Nickshelley@openwater.uk.com
Registered Office
OpenWater International Ltd
The Manse Station Road,
Plumpton Green, Lewes,
East Sussex,
England
BN7 3BX
11250848 Registered in England & Wales
